PIELDS ENGINEERING
Hydrogen Production Facilities
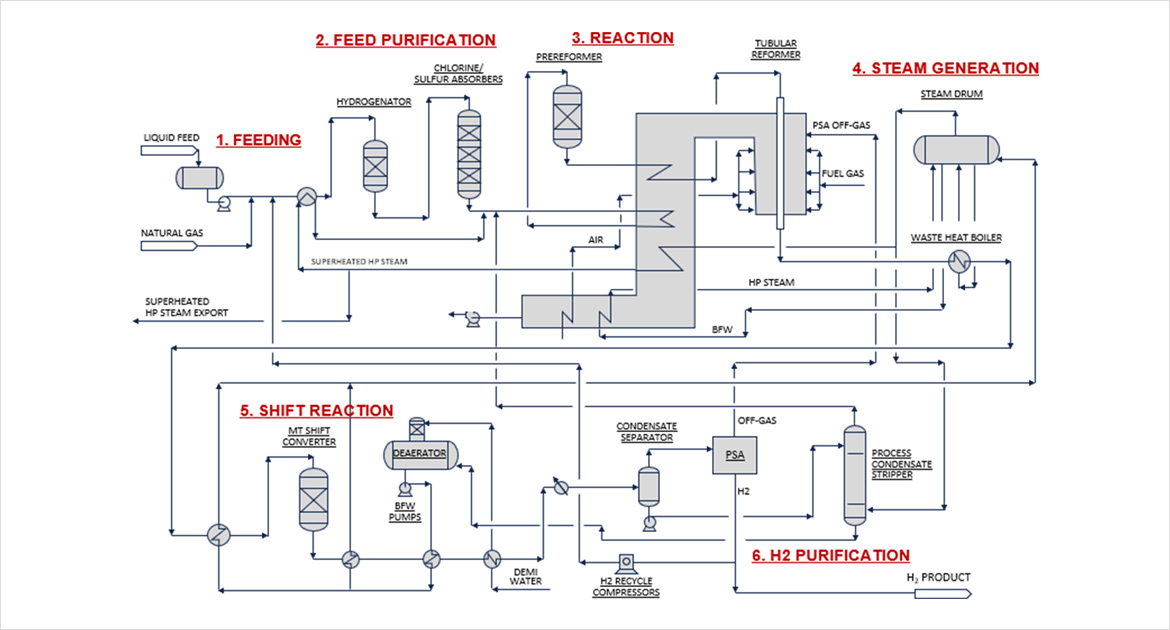
SMR (STEAMMETHANE REFORMING)
Steam Methane Reforming (SMR) is the most common and widely applied process used for hydrogen production.
It produces hydrogen based on the reaction between natural gas (methane) and water vapor (steam) at high temperatures between 700 and 1000°C.
This process accounts for more than 50% of the world's hydrogen production.
When methane is injected with hot steam, it first separates into hydrogen and carbon monoxide through a water-gas shift reaction.
Further hydrogen is generated through the dissociation of carbon monoxide in the High Temperature Shift (HTS) process using a catalyst.
High-purity hydrogen is then produced through the Pressure Swing Adsorption (PSA) process.
CH4 + H2O <-> 3H2 + CO
CO + H2O<-> H2 + CO2
Technical Services from Pields Engineering
-

step1
Process Design
(Basic & Detailed) -

step2
Technical Consulting for
Factory Optimization and
Performance Improvement -

step3
Factory Renovation
-

step4
Feasibility Studies
-

step5
Training
(Operations and
Operator Training) -

step6
Commissioning
Support
Benefits of Pields Engineering
- Provides basic design services as part of an EPC package.
- Experienced in securing key package vendors and collaborating on EPC work.
- Applies proven design packages quickly to shorten the overall project schedule to the minimum.
- The rapid response of experienced personnel helps reduce costs and time.
- The use of standardized designs, materials, and construction methods helps lower costs and time.
- Experienced in working on projects across multiple disciplines.
- Reduces CO2 emissions with CCUS technology.

